Upregulation of Protein Kinase C Gamma in Spinal Cord after Formalin Intracolonic Injection
Chen Chen, Jingjin Li, Peter Fong, Guo Shao and Kerui Gong.
DOI10.4172/2472-1913.100005
Chen Chen1,2,Jingjin Li2, Peter Fong3,Guo Shao4 and Kerui Gong2,5*
1Taiwan Center for Disease Control and Prevention, Taiwan, Shandong Province, China
2Department of Neurobiology, Capital Medical University, Beijing, China
3Department of Neurology, University of California San Francisco, San Francisco, CA, USA
4Biomedicine Research Center and Basic Medical School, Baotou Medical College, Baotou, Inner Mongolia, China
5Department of Oral and Maxillofacial Surgery, University of California San Francisco, San Francisco, CA, USA
- *Corresponding Author:
- Kerui Gong
Department of oral and maxillofacial surgery, 533 Parnassus Avenue, Room UB10
Campus Box 0440, University of California San Francisco
San Francisco, California 94143, USA
Tel: +1-415-502-7406
E-mail: keruigong@gmail.com
Received date: October 24, 2015; Accepted date: January 04, 2016; Published date: January 11, 2016
Citation: Chen C, Li J, Fong P, et al. Upregulation of Protein Kinase C Gamma in Spinal Cord after Formalin Intracolonic Injection. Headache Pain Manag. 2016, 1:1.
Copyright: © 2016 Chen C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Visceral pain remains one of the most commonly observed clinical symptoms, and yet, it continues to represent a large unmet therapeutic demand. Work from our lab and others have aimed to describe potential mechanisms underlying the pathophysiology of visceral pain and have identified protein kinase C gamma (PKC γ) as an important molecular target, as it undergoes prominent membrane translocation in visceral pain and directly affects many pain signaling pathways. In this study, we continued our investigation of PKC γ modulation in visceral pain and measured PKC γ expression in the spinal cord at 30, 60, and 120 min following visceral pain that was induced by intracolonic injection of formalin. PKC γ mRNA was significantly increased at 60 min following formalin injection, but not at 30 or 120 min following injection, as detected by reverse transcriptase polymerase chain reaction (RT-PCR). Western blot analysis and immunocytochemistry confirmed the up-regulation of PKC γ protein expression at 60 min following formalin injection. Furthermore, immunocytochemistry also confirmed the localization of PKC γ positive neurons to the superficial layers of the spinal cord dorsal horn, where primary afferent terminals transmitting pain information are received. Taken together, our results implicate an important role for PKC γ in the development of visceral pain.
Keywords
Visceral pain; Protein kinase C; Formalin; Western blot; RT-PCR
Introduction
Visceral pain is one of the most common reasons for which people seek medical treatment, but it does not respond adequately to current analgesic treatment strategies. Numerous researches have indicated that the pathophysiology of visceral pain is distinct from that of somatic pain [1] suggesting that visceral pain and somatic pain are mediated by different mechanisms and may respond to distinct molecular treatment targets. In contrast with somatic pain, visceral pain is diffuse, difficult to localize and often referred to a distal somatic area [2]. Thus, current research describing somatic pain may not be directly applicable to visceral pain, and further elucidation of the mechanisms that mediate visceral pain is necessary in order to identify appropriate molecular targets to improve the clinical treatment of visceral pain.
Central sensitization—a phenomenon in which changes in central nervous system (CNS) neurons result in abnormal pain sensitivity to noxious and innocuous stimuli [3] — has been regarded as one of the most important mechanisms in maintaining pain [4]. In addition, it has also been thought to be critical in the development of visceral pain [5]. The first neuronal synapse in the transduction of pain occurs in the dorsal horn of the spinal cord, where afferent fibers from dorsal root ganglia (DRG) are received. Therefore, dorsal horn serves as the gate for pain information relay, and it is one of the main anatomical sites in central sensitization. And it has been demonstrated that neurons in dorsal horn experience hyper-excitability in central sensitization induced by pain stimuli [6].
While protein kinase C (PKC) has been widely implicated in central sensitization that is induced by somatic pain [7-9], its role in central sensitization induced by visceral pain has only recently been investigated [10, 11]. PKC includes 11 isozymes that are categorized into 3 groups: cPKCs (α, βI, βII, γ), nPKCs (ε, δ) and aPKC (ξ) [12]. Among these, PKC γ is regarded as the main subtype in mediating pain transduction and is also critically positioned in the spinal cord to relay pain information. In the rat spinal cord, PKC γ is most densely distributed throughout layer II, where dorsal horn neurons also receive DRG afferent inputs relaying pain information [11]. Our previous work also demonstrated that visceral pain in rats induced by intracolonic formalin injection resulted in significantly increased PKC γ membrane translocation, indicating that PKC γ activity is augmented following visceral pain [10]. However, this previous study was unable to conclude if visceral pain also increases PKC γ protein synthesis. In the present study, we investigated the effects of visceral pain induced by intracolonic formalin injection in modulating PKC γ mRNA and protein expression.
Materials and Method
Animal model
Experiments were conducted with adult male Wistar rats (weighing 200–250 g) that were housed individually with food and water available ad libitum and were maintained in temperature controlled (23-25°C) and light-controlled conditions (light/dark cycle, 12 h/12 h). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Capital Medical University and were consistent with guidelines provided by the International Association for the Study of Pain. All efforts were made to minimize animal pain and distress and the number of animals used. Rats were randomly divided into 3 groups: (1) Naïve rats (N), (2) Intracolonic (I.C), Saline (S) Injection, and (3) Intracolonic formalin (F) Injection.
Visceral pain model
Visceral pain was induced in animals as previously described [10, 13]. Briefly, after cleaning the contents of the colon, a colonoscope was inserted through the anus. Then, 100 μl of either 5% formalin or saline was injected into the colon under visual aid. Care was taken to ensure that formalin was injected into only the submucosal layer of the colon.
RT-PCR
At 30, 60, and 120 min following intracolonic injection, rats were sacrificed by intraperitoneal (I.P) injection of a lethal dose of sodium pentobarbital (50 mg/kg). These experimental time points were chosen based upon our previous work indicating that the greatest changes in pain behaviors and PKC γ membrane translocation occur at 30, 60, and 120 min following formalin injection [10].
After decapitation, spinal cords were flushed with cold saline, followed by dissection of the L6-S1 segments. Total RNA was isolated with Qiagen RNeasy mini kit (Qiagen, Valencia, CA). RNA integrity was confirmed by electrophoresis through a 1% agrose gel with formaldehyde. A mixture of 2 μg of RNA, 1 μL of random primer (0.125 g/L), 2 μl of DTT, and ribonuclease-free water was added into a tube to a final volume of 12 μl. Tubes were then heated to 70°C for 10 minutes, placed on ice, and then mixed with the addition of 4 μl of ribonuclease-free water, 4 μL of 5X reverse transcriptase (RT) buffer, 1 μL of 10 mmol/L deoxynucleoside triphosphates, 2 μl of 0.1 mol/L dithiothreitol, 1 μl of RNasin (40 U/L; Promega, Pittsburgh,PA), and 1 μl of superscript II reverse transcripase (108 U/L; Invitrogen, Carlsbad, CA).
The RT reaction was performed at 42°C for 50 minutes and at 70°C for 15 minutes. Tubes were then left on ice for polymerase chain reaction (PCR). The primers used for the PCR of the PKC γ gene were as follows: forward primer 5′-CAGGACAGCCACCCTTTGAT-3, reverse primer 5′-GAACACCTAGCGGCAGCAGA-3’. The primers for GAPDH were as follows: forward primer 5′-CCCTTCATTGACCTCAAC-3′, reverse primer 5′-TTCACACCCATCACAAAC-3′.
The PCR reaction contained 10.3 μl of sterile water, 2 μl of 10 X PCR buffer, 1.5 μl of 10 mmol/L deoxynucleoside triphosphates, 2 μl of each forward and reverse primer, 0.2 μl of Taq DNA polymerase (5 × 106 u/L; Qiagen, Valencia, CA), and 2 μl of the RT reaction mixture. The PCR conditions for amplification of PKC γ were as follows: initial denaturation for 5 minutes at 94°C, followed by 3-step cycling: denaturation for 40 s at 94°C, annealing for 40 s at 50°C, and extension for 45 s at 72°C (32 cycles), followed by final extension for 7 minutes at 72°C. The conditions for amplification of GAPDH were as follows: initial denaturation for 3 minutes at 94°C, followed by 3-step cycling: denaturation for 40 s at 92°C, annealing for 40 s at 50°C, and extension for 45 s at 72°C (20 cycles), followed by final extension for 7 minutes at 72°C. Products were resolved by 1.2% agarose. Quantification of the RT-PCR bands was performed using a phosphorimager (Quantity one Gel doc program; Bio-Rad, Hercules, CA), and results were measured as the ratio of PKC γ to GAPDH for each animal group.
Western blot
Rats were sacrificed by I.P. injection of a lethal dose of sodium pentobarbital (50 mg/kg) 60 min following formalin injection. Spinal cords were dissected in the same way as described in RTPCR. Dissected spinal cord tissues were immediately homogenized at 4°C in lysis buffer, containing 50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1 mM ethylenediamine-N,N,N’,N’-tetraacetic acid (EDTA), 0.5% Triton X-100, and a phosphatase inhibitor cocktail (Cat # P0044, Sigma-Aldrich, St. Louis, MO).
Protein concentrations of each sample were determined using a BCA kit (Pierce, Rockford, Ill., USA), before equal loading the same amount of protein into wells of 10% SDS-PAGE gels for electrophoresis. Protein was transferred to nitrocellulose membranes (Schleicher and Schell, USA). The membrane was rinsed for 10 min. with TTBS (20 mM Tris-Cl, pH 7.5, containing 0.15 M NaCl and 0.05% Tween-20) and incubated with blocking solution with 10% nonfat milk in TTBS for 2 hr at room temperature. Membranes were subsequently incubated with rabbit anti-PKC γ polyclonal antibody (1:1,000, Santa Cruz Biotechnology, USA) and β-actin monoclonal antibody (1:1,000, Santa Cruz Biotechnology, USA) for 3 h at room temperature, rinsed 3 times (10 min each) in TTBS, then incubated with horse radish peroxidase-conjugated goat anti-rabbit IgG (1:5,000, Amersham, USA) for 1 h at room temperature, and rinsed 3 times again (10 min each) in TTBS. Finally, membranes were incubated in Super signal West chemiluminescent reagents (Pierce, USA) to obtain a signal for exposure to radiographic film. Immunoblot images were scanned and densitometric analysis was performed (ImageQuant, Amersham Biosciences, Piscataway, N.J, USA). Results were measured by normalizing PKC γ bands to β-actin bands.
Immunocytochemistry
Rats were anesthetized with i.p. injection of sodium pentobarbital (50 mg/kg) and intracardially perfused with phosphate-buffered saline (PBS), then 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. L6–S1 segments of the spinal cord were removed and post-fixed in the same fixative overnight and then transferred to 30% sucrose in 0.1 M PB for cryoprotection. Cryostat sections (30 μm) were collected in the coronal plane, and sections were processed for immunocytochemistry. Briefly, sections were incubated for 1h at room temperature in blocking buffer (1% Bovine Serum Albumin), then incubated overnight at 4oC with rabbit anti-PKC γ primary antibodies (1:1000, Santa Cruz Biotechnology, USA). Sections were then rinsed 3 times in PBS with 1% Triton (PBST), incubated for 2 hours at room temperature with goat anti-rabbit immunoglobulin G conjugated to biocytin (1:300, Molecular Probes, Eugene, OR). After rinsing 3 times in PBST, sections were incubated with streptavidin conjugated to HRP (1:300) at room temperature for 1 h. Then, they were rinsed twice with PBST, incubated with DAB working solution (Vector laboratories, CA) for 10 min, and rinsed for 5 min in water. Images were viewed and captured using a Nikon EF600 microscope. Negative control experiments were performed by omitting the anti-PKC γ primary antibody.
Data analysis
All data are expressed as means ± SEM, and statistical analysis was conducted with one-way ANOVA, followed by post hoc fisher’s PLSD, unless specified. P-values less than 0.05 were considered statistically significant.
Results
Intracolonic formalin injection increased PKC γ mRNA expression at 60 min post injection
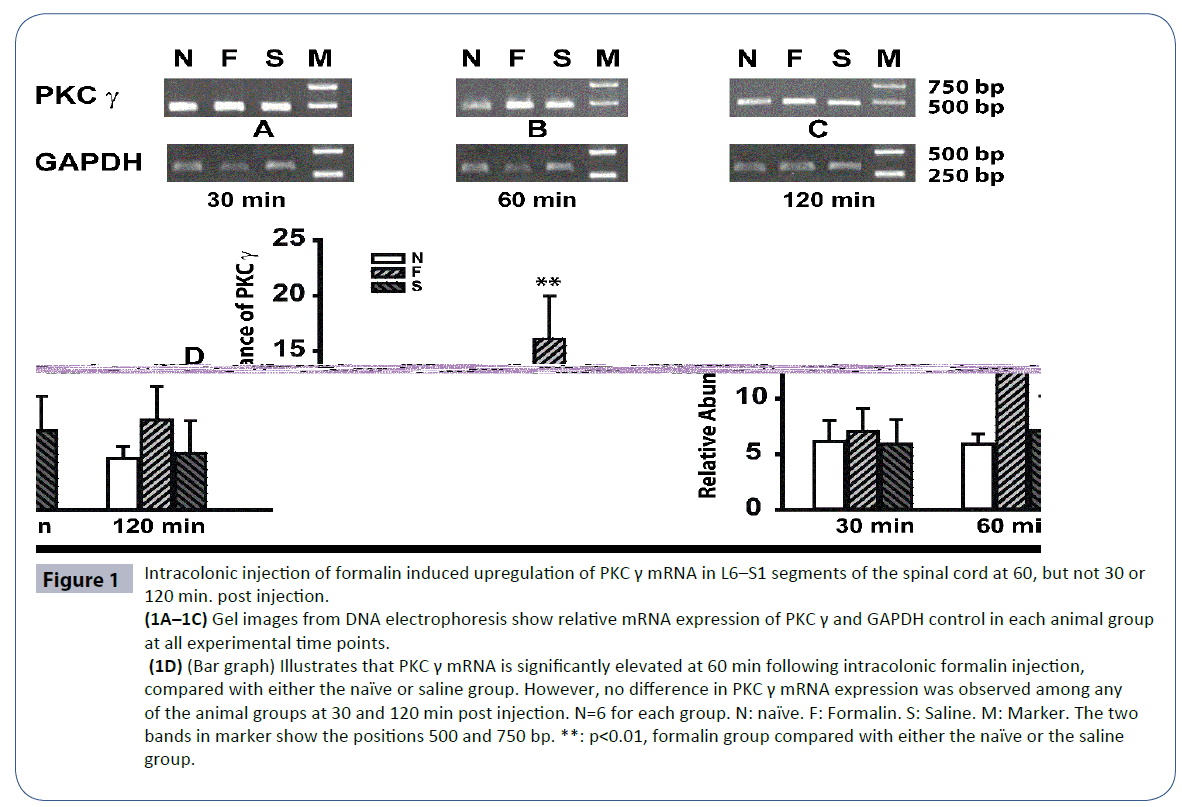
PKC γ mRNA expression levels were detected in each of the 3 animal groups and at all of the experimental time points (30, 60, and 120 min post injection). At 30 min post injection, PKC γ mRNA expression in the naïve, saline, and formalin groups were 6.1 ± 1.9, 5.9 ± 2.2, and 7.0 ± 2.1, respectively (Figure 1A). Statistical analysis did not reveal differences between any of the groups, indicating that PKC γ mRNA was not up-regulated within the first 30 min after formalin injection (Figure 1A). At 60 min following injection, PKC γ mRNA expression in the saline group (7.1 ± 3.1) was comparable with that measured at 30 min post injection. However, PKC γ mRNA expression in the formalin group increased significantly to 16.1 ± 4.9—more than twice the expression levels of either the naïve or the saline group at 60 min post injection (p<0.01), (Figures 1B and 1D). At 120 min after intracolonic injection, PKC γ mRNA levels decreased to 8.1 ± 3.0 in the formalin group and to 4.6 ± 1.1 and 5.1 ± 2.9 in the naïve and saline groups, respectively (Figures 1C and 1D).
Figure 1 Intracolonic injection of formalin induced upregulation of PKC γ mRNA in L6–S1 segments of the spinal cord at 60, but not 30 or 120 min. post injection. (1A–1C) Gel images from DNA electrophoresis show relative mRNA expression of PKC γ and GAPDH control in each animal group at all experimental time points. (1D) (Bar graph) Illustrates that PKC γ mRNA is significantly elevated at 60 min following intracolonic formalin injection, compared with either the naïve or saline group. However, no difference in PKC γ mRNA expression was observed among any of the animal groups at 30 and 120 min post injection. N=6 for each group. N: naïve. F: Formalin. S: Saline. M: Marker. The two bands in marker show the positions 500 and 750 bp. **: p<0.01, formalin group compared with either the naïve or the saline group.
Intracolonic injection increased protein expression of PKC γ
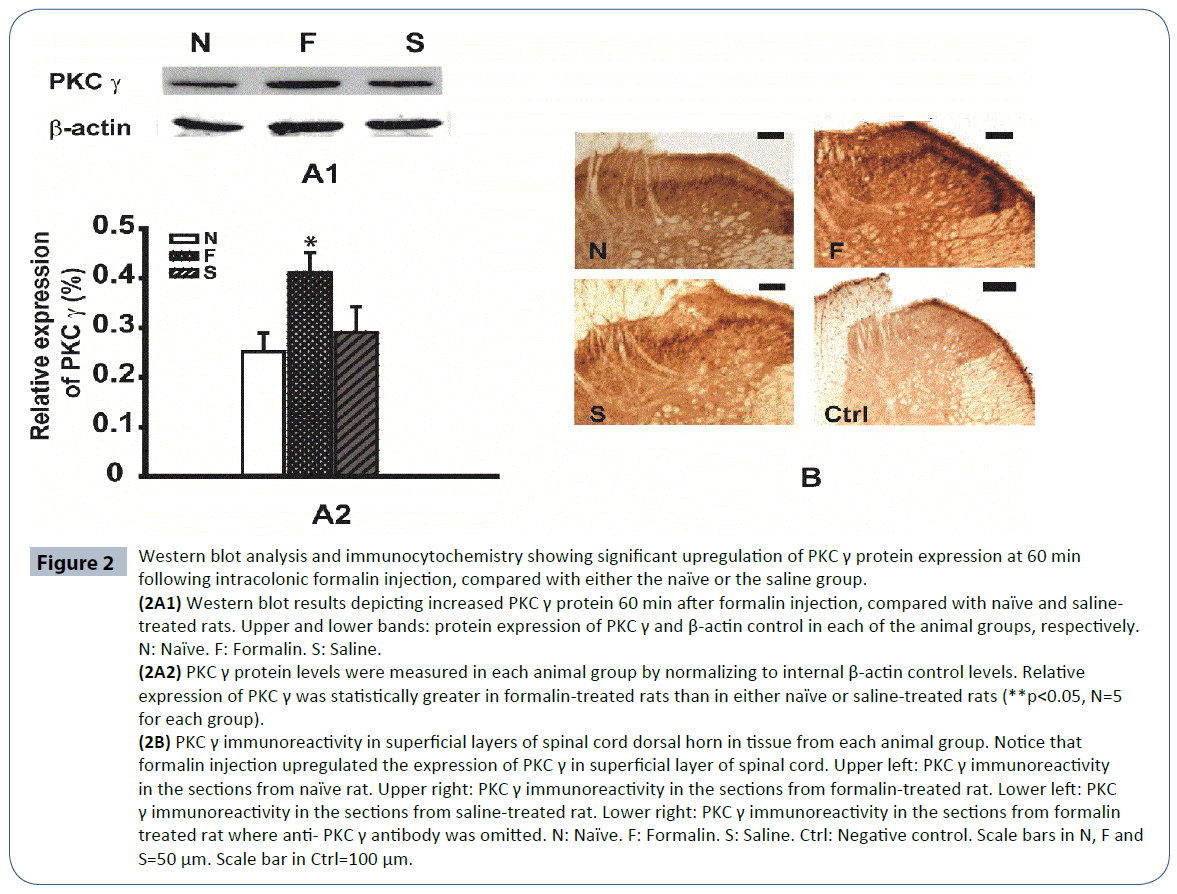
Our results from RT-PCR indicated that PKC γ mRNA levels were upregulated at 60 min following formalin injection, compared with the other experimental time points. Therefore, we examined the protein expression of PKC γ at the same experimental time point. At 60 min following formalin injection, PKC γ protein expression in the naïve and saline-treated rats were 0.25 ± 0.03 and 0.29 ± 0.05, respectively, but were significantly greater in the formalin-treated rats (0.41 ± 0.04, Figure 2A2, p<0.05, n=6).
Figure 2 Western blot analysis and immunocytochemistry showing significant upregulation of PKC γ protein expression at 60 min following intracolonic formalin injection, compared with either the naïve or the saline group.
(2A1) Western blot results depicting increased PKC γ protein 60 min after formalin injection, compared with naïve and salinetreated rats. Upper and lower bands: protein expression of PKC γ and β-actin control in each of the animal groups, respectively. N: Naïve. F: Formalin. S: Saline. (2A2) PKC γ protein levels were measured in each animal group by normalizing to internal β-actin control levels. Relative expression of PKC γ was statistically greater in formalin-treated rats than in either naïve or saline-treated rats (**p<0.05, N=5 for each group). (2B) PKC γ immunoreactivity in superficial layers of spinal cord dorsal horn in tissue from each animal group. Notice that formalin injection upregulated the expression of PKC γ in superficial layer of spinal cord. Upper left: PKC γ immunoreactivity in the sections from naïve rat. Upper right: PKC γ immunoreactivity in the sections from formalin-treated rat. Lower left: PKC γ immunoreactivity in the sections from saline-treated rat. Lower right: PKC γ immunoreactivity in the sections from formalin treated rat where anti- PKC γ antibody was omitted. N: Naïve. F: Formalin. S: Saline. Ctrl: Negative control. Scale bars in N, F and S=50 μm. Scale bar in Ctrl=100 μm.
Immunocytochemistry was performed in L6–S1 spinal cord sections from rats sacrificed at 60 min post injection to further characterize PKC γ protein expression and to determine the anatomical distribution of PKC γ in the spinal cord. PKC γ immunopositive cells were identified in tissue from rats in the naïve, saline-treated, and formalin-treated groups. Our results showed that while PKC γ immunoreactivity in the naïve and saline groups were comparable, immunostaining in tissue from formalin-treated rats showed much greater PKC γ expression. Furthermore, PKC γ-containing neurons were localized to the superficial layers of the spinal cord dorsal horn. Negative control experiments were also performed with the omission of the anti-PKC γ antibody in order to confirm immunospecificity of our reagents and methods. These data, combined with our results from RT-PCR and western blot analysis, indicate that both PKC γ mRNA and PKC γ protein expression are significantly upregulated by 60 min following visceral pain (Figures 2A1, 2A2 and 2B).
Discussion
In the present study, we provided multifold evidence that robust upregulation of PKC γ occurs 60 min after the induction of visceral pain. In addition, our methods—examining both the mRNA and the protein expression of PKC γ through RT-PCR, western blot, and immunocytochemistry—provide thorough evidence demonstrating that visceral pain markedly increases PKC γ protein synthesis. These data, in light of our previous work indicating that PKC γ membrane translocation is also increased in visceral pain [10], strongly implicate an important role for increased PKC γ activity in the pathophysiology of visceral pain.
Intracolonic injection of formalin has been widely utilized as a robust animal model of visceral pain [10, 13]. Utilizing this model, we revealed that PKC γ mRNA and protein expression is sharply elevated at 60 min following visceral pain induction. In our previous study, we showed that intracolonic formalin injection resulted in increased PKC γ membrane translocation as early as 30 min post injection, but not as late as 60 or 120 min post injection [10]. The differences in the time courses of the increase in PKC γ membrane translocation, compared with that of the increase in PKC γ mRNA and protein expression, indicate that PKC γ undergoes at least two different mechanisms of modulation in visceral pain—activation by translocation to the membrane and de-novo protein synthesis.
While the time courses of PKC γ membrane translocation and PKC γ protein synthesis following formalin injection do not align, their differences may represent two different components of visceral pain that is supported by behavioral data from our previous work. In our previous study, we observed that animals exhibited the most prominent pain behaviors 30 min after intracolonic formalin injection, which is when we also observed the greatest increase in PKC γ membrane translocation [10]. At 60 min post injection, animals continued to exhibit obvious, albeit less pronounced and less frequent, pain behaviors. In conjunction with our current data, indicating that PKC γ protein expression is significantly elevated at 60 (but not at 30 or 120) min following formalin injection (Figures 1 and 2), these data suggest that PKC γ membrane translocation may play a critical role in the initiation of visceral pain, while PKC γ protein synthesis may be involved in the subsequent maintenance of visceral pain. Considering the kinetics of membrane translocation, in comparison with those of mRNA synthesis, it is reasonable to expect that PKC γ membrane translocation should reach a maximum before PKC γ protein expression does.
Works from others have similarly reported that PKC membrane translocation is significantly increased in other pain models, including both visceral pain and somatic pain [10, 12, 14, 15]. Moreover, work from He et al. have suggested that PKC γ is involved in the initiation of visceral pain induced by post-traumatic stress disorder (PTSD) [11]. However, this report also emphasized that while PKC γ is important for the initiation of visceral pain, PKC ε is critical for the initiation and maintenance of visceral pain. One plausible explanation for the discrepancy of these results with our data might be that the duration of visceral pain hypersensitivity alters how much PKC γ and PKC ε contributes to the maintenance of pain. Behavioral data from our previous work indicated that pain behaviors subsided 75 minutes after intracolonic formalin injection [16]. In contrast, animals with visceral pain induced by PTSD continue to exhibit visceral pain hypersensitivity for nearly one month following the induction of pain [11]. Thus, it is possible that PKC gamma contributes to the initiation and maintenance of acute visceral pain, while chronic visceral pain results in a shift towards PKC epsilon contributing more critically to the initiation and maintenance of prolonged pain.
Our results indicating that PKC γ immune-positive cells were found primarily in the superficial layers of the spinal cord dorsal horn are also consistent with previous reports in the literature describing the anatomical distribution of PKC γ [17, 18]. The superficial layers of the spinal cord dorsal horn, which classically include layers I and II, receive inputs from peripheral afferent fibers and are the most critical anatomical site for receiving pain information [19]. Thus, our results, confirming that PKC γ cells are localized to layers I and II of the spinal cord, provide important evidence that the PKC γ modulations we observed are related to nociceptive activity. Furthermore, our results provide an independent source of evidence to support our observations from RT-PCR and Western blot analysis of the up-regulation of PKC γ mRNA and protein synthesis in visceral pain (Figure 2B).
In the spinal cord, the majority of the PKC γ positive neurons have been shown to be excitatory [18], and PKC activation, in turn, promotes neuronal excitability [20]. Thus, our data suggest that up-regulation of PKC γ in visceral pain may promote excitatory activity in neurons transmitting pain information to higher levels of pain processing, resulting in not only the perception of visceral pain, but also in central sensitization, a critical mechanism underlying persistent pain [6, 21]. In many pain models, PKC has been found to be necessary for maintaining central sensitization and directly affects molecular signaling pathways mediating central sensitization in pain. Activation of PKC results in the direct activation of intracellular signaling molecules of the mitogenactivated protein kinase (MAPK) family that are critically involved in pain sensitization, including extracellular-signal-related kinase (ERK) 1 and 2, p38, and stress-activated protein kinase (SAPK)/ c-Jun N-terminal kinase (JNK) [4, 22, 23]. In addition, PKC γ has also been reported to phosphorylate NMDA receptors [24] and promote the trafficking of NMDA receptors to the cell membrane [25]. NMDA receptors have been shown to also be up-regulated in visceral pain [26] and are widely implicated in pain processing pathways. Thus, it is important to note that the modulations of PKC γ expression or activity may generate downstream effects that affect not only visceral pain, but also a broad spectrum of pain signaling pathways. In summary, our present findings provide further evidence implicating the important role of PKC γ in visceral pain that are in support of previous work from our lab and in the literature. Importantly, our results also demonstrate that visceral pain promotes not only the increased membrane translocation of PKC γ, but also the active synthesis of new PKC γ proteins that may result in downstream activation of upper level pain signaling pathways.
Acknowledgement
This work was supported by the Scientific Developing Program of Beijing Municipal Commission of Education (01KJ2092, KM200410025004) to Jingjin Li.
Conflicts of Interest
There are no conflicts of interest.
References
- Cervero F, Laird JM (1999) Visceral pain. Lancet 353:2145-2148.
- SikandarS, Dickenson AH (2012) Visceral pain: the ins and outs, the ups and downs. CurrOpin Support Palliat Care 6:17-26.
- Latremoliere A, Woolf CJ (2009) Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10:895-926.
- Xiaoping G, Xiaofang Z, Yaguo Z, Juan Z, Junhua W, et al. (2010) Involvement of the spinal NMDA receptor/PKCgamma signaling pathway in the development of bone cancer pain. Brain Res 1335:83-90.
- Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152:S2-15.
- Woolf CJ (2007) Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology 106:864-867.
- Fang L, Wu J, Lin Q, Willis WD (2003) Protein kinases regulate the phosphorylation of the GluR1 subunit of AMPA receptors of spinal cord in rats following noxious stimulation. Brain Res Mol Brain Res 118:160-165.
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, et al. (2004) Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci 24:8310-8321.
- Sikand P, Premkumar LS (2007) Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J Physiol 581:631-647.
- Zhang Y, Gong K, Zhou W, Shao G, Li S, et al. (2011) Involvement of subtypes gamma and epsilon of protein kinase C in colon pain induced by formalin injection. Neurosignals 19:142-150.
- He YQ, Chen Q, Ji L, Wang ZG, Bai ZH, et al. (2013) PKCgamma receptor mediates visceral nociception and hyperalgesia following exposure to PTSD-like stress in the spinal cord of rats. Mol Pain 9:35.
- Newton AC (1997) Regulation of protein kinase C. CurrOpin Cell Biol 9:161-167.
- Miampamba M, Chery-Croze S, Gorry F, Berger F, Chayvialle JA (1994) Inflammation of the colonic wall induced by formalin as a model of acute visceral pain. Pain 57:327-334.
- Mao J, Price DD, Mayer DJ, Hayes RL (1992) Pain-related increases in spinal cord membrane-bound protein kinase C following peripheral nerve injury. Brain Res 588:144-149.
- Wang Y, Wu J, Guo R, Zhao Y, Zhang M, et al. (2013) Surgical incision induces phosphorylation of AMPA receptor GluR1 subunits at Serine-831 sites and GluR1 trafficking in spinal cord dorsal horn via a protein kinase Cgamma-dependent mechanism. Neuroscience 240:361-370.
- Zhang YB, Guo ZD, Li MY, Fong P, Zhang JG, et al. (2015) Gabapentin Effects on PKC-ERK1/2 Signaling in the Spinal Cord of Rats with Formalin-Induced Visceral Inflammatory Pain. PLoS One 10:e0141142.
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI (1997) Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science 278:279-283.
- Polgar E, Fowler JH, McGill MM, Todd AJ (1999) The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res 833:71-80.
- Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267-284.
- Astman N, Gutnick MJ, Fleidervish IA (1998) Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ current in mouse neocortical slices. J Neurophysiol 80:1547-1551.
- Farrell KE, Callister RJ, Keely S (2014) Understanding and targeting centrally mediated visceral pain in inflammatory bowel disease. Front Pharmacol 5:27.
- Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, et al. (2008) Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci 28:4533-4540.
- Di CesareMannelli L, Ghelardini C, Toscano A, Pacini A, Bartolini A (2010) The neuropathy-protective agent acetyl-L-carnitine activates protein kinase C-gamma and MAPKs in a rat model of neuropathic pain. Neuroscience 165:1345-1352.
- Roh DH, Choi SR, Yoon SY, Kang SY, Moon JY, et al. (2011) Spinal neuronal NOS activation mediates sigma-1 receptor-induced mechanical and thermal hypersensitivity in mice: involvement of PKC-dependent GluN1 phosphorylation. Br J Pharmacol 163:1707-1720.
- Yan JZ, Xu Z, Ren SQ, Hu B, Yao W, et al. (2011) Protein kinase C promotes N-methyl-D-aspartate (NMDA) receptor trafficking by indirectly triggering calcium/calmodulin-dependent protein kinase II (CaMKII) autophosphorylation. J BiolChem 286:25187-25200.
- Luo XQ, Cai QY, Chen Y, Guo LX, Chen AQ, et al. (2014) Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord contributes to chronic visceral pain in rats. Brain Res 1542:167-175.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences