The Headaches and Polymorphisms of the Methylenetetrahydrofolate Reductase
Elisabetta Tozzi
DOI10.4172/2472-1913.100042
Elisabetta Tozzi, Alessandra Piccorossi, Cristina Gammella, Agnese Onofri, Alberto Verrotti and Enzo Sechi*
Neuropsychiatric Clinic Department of life, health and environmental science(MESVA) University of L'Aquila, Italy
- *Corresponding Author:
- Elisabetta Tozzi
Neuropsychiatric Clinic - Headache center Department of life, health and environmental science
University L'Aquila, Italy
Tel: 0039-0862-433662
E-mail: elisabetta.tozzi@univaq.it
Received Date: August 27, 2017; Accepted Date: October 04, 2017; Published Date: October 10, 2017
Citation: Tozzi E, Piccorossi A, Gammella C, Onofri A, Verrotti A, et al. (2017)The Headaches and Polymorphisms of the Methylenetetrahydrofolate Reductase. J Headache Pain Manag 2:12. doi: 10.4172/2472-1913.100042
Abstract
The headaches are a heterogeneous clinical manifestations characterized not only by pain but also by a severe multifactorial disabilities. Numerous studies showed the association between the enzyme MTHFR mutations, the T allele and genotype TT and migraine, particularly migraine with aura.
Aim of the study: On the basis of the literature data we propose to evaluate the prevalence of the MTHFR mutation in the population of patients admitted to a headache center of the childhood.
Materials and methods: we examined 226 children, aged 5-17 years, admitted to " Headache Center" of Children Neuropsychiatry of Hospital San Salvatore L'Aquila, from 2013 to the year 2015. The diagnosis of headache is made according to ICHD III criteria. The identification of the MTHFR C677T polymorphism of the enzyme was carried out by the Real-time PCR qualitative. The statistical analysis by SAS System.
Results: Diagnosis of migraine without aura in 96 patients, migraine with aura in 39, in 18 chronic migraine, frequent episodic tension-type headache in 40, chronic tension type headache in 20 and in 13 other headaches. 61 patients are without mutation, 85 with heterozygous mutation, and 28 homozygous. Therefore, the mutation is present in 147 patients with a percentage of 65%. The heterozygous mutation is present in 50.56% of females and 47.06% males; the homozygous in 10.19% and 12.94% in females than males. In migraineurs the mutation is present in 66.67% compared with patients with tension-type headache (33.33%), homozygous is the 70.37% versus 29.63% (p<0.001). Homocysteine levels are elevated only on the 8.69% of mutated patients, of which 5/12 patients have a mutation in homozygosity.
Conclusion: we confirme the statistical association between MTHFR mutation both in the heterozygous and homozygous forms and migraines. The migraines with aura, unlike the literature, does not seem to have an important role.
https://marmaris.tours
https://getmarmaristour.com
https://dailytourmarmaris.com
https://marmaristourguide.com
https://marmaris.live
https://marmaris.world
https://marmaris.yachts
Keywords
Headache; Migraine; Children; C677T polimorphism
Introduction
Migraine is a common debilitating disorder associated with multiple symptoms and not only with the pain. The migraines are on a chapter of “The International Headache Society classification, 2013 version” [1] and were considered two different categories: migraine with aura (MA) and migraine without aura (MO). The MA has been related to Methylenetetrahydrofolate Reductase (MTHFR) gene mutation and high levels of Homocysteine (Hcy) [2-8]. Hcy is a product of methionine metabolism and is a sulfur-containing amino acid [2-4]. MTHFR is an enzyme responsible for the conversion of Hcy to methionine and it catalyzes the reduction of 5,10-methylenetetrahydrofolate (CH2-THF) to 5-methylenetetrahydrofolate [4]. If Hcy is not properly metabolized, its high levels could be associated with vascular damage and endothelial remodelling, this condition could be responsible of a reduction in blood oxygenation in brain circulation close to onset and maintenance of migraine attacks [5,6]. The polymorphism most frequently identified is C677T and it is related not only to migraine, but also to other neurological/ psychiatric and organic symptoms as neural tube defects, cerebrovascular and cardiovascular disease, hypertension, glaucoma. Among neurological and psychiatric disorders that are associated with migraine, depression, anxiety disorders, epilepsy and sleep disorders are reported. More recently, an association with cardiovascular disease has been highlighted, particularly with ischemic stroke [4-7]. C677T polymorphism is characterized by a substitution of an alanine with avaline at position 222; this substitution leads to a reduction on enzyme activity [6]. Subjects with the C677T variant present reduced capacity to remethylate homocysteine to methionine, these conditions increase homocysteine plasma levels [6,8]. In most of the genetic determination of C677T polymorphism studies carried out on whole blood samples using EDTA tubes and transferred in an upright position to local lab, where were stored at -20°C to freezer by the Real-Time Polymerase Chain Reaction (RT- PCR), and are considered the homozygous and heterozygous T alleles of variant [9]. The Total Homocysteine (tHcY) was determined using a Chemi-Luminescent Immunoenzymatic Assay (CMIA) Homocysteine Architect [10]. The data of Italy population show mutation in homozigous in 8, 8-10% of people [11,12]. According to Lea [2] and other several Authors, [6,11] C677T MTHFR variant predisposes to a susceptibility to migraine with aura and not to that without aura, in particular, it is well- known for European Caucasian population. A meta-analisys of 15 case control studies showed a close correlation between TT variant and Caucasians suffering from MA, in non-Caucasian population this genotype is associated with total migraine [11]. In a study of Bottini et al. was analyzed the prevalence of homozygous variant of an Italian study population, it was identified a trend towards an increased incidence of migraine in subjects who carried the mutation [8]; another study examined a group of Croatian pediatric patients suffering from migraine, the 677TT genotype was associated with an higher risk of migraine [11]. No association was found in Spanish and Portuguese adult migraineurs [13,14]. Lorenz et al analyzed MTHFR 677TT polymorphism in a group of Estonian pediatric migraineurs and did not detect a close link with migraine prevalence [14]. So MTHFR C677T variant differently occur in different countries [15]. In the pathogenesis of aura migraine is hereby accepted the concept of “cortical spreading depression (CSD)”, in the aura phase there is a cerebral oligoemia that starts to spread anteriorly and a condition of hyperemia follows to this [16,17]. It is known that about 37% of migraineurs presents white matter abnormalities, predominantly in the posterior circulation and in cerebellar territory; some case studies show a prevalence of these lesions (61%) in people with aura migraine and female [18]. Among the causes of such anomalies, in addition to the patent foramen ovale, high blood pressure and other risk factors for stroke, such as cigarette smoking, use of oral contraceptives, obesity, hypercholesterolemia, there is the MTHFR C677T mutation. An elevated plasma vWF activity is associated with MTHFR TT genotypes, so it represents a connection between migraine and ischemic predisposition [13,18]. A case study conducted in pediatric patients suffering from stroke, confirms the presence of hyperhomocysteinemia among the causal factors of stroke [19-21]. According to recent publications, migraine headache, especially those with aura, however, it could be conceived as the pathological mediator between the mutation and the phenotypic outcome of stroke [11,13]. In a study of Lea et al. on 52 patients suffering from aura migraine with increased levels of homocysteine, it was administered to patients vitamin supplementation, as homocysteine levels decreased frequency and severity of migraine attacks declined [22] In the trial of Di Rosa et al. on 16 children with MA and high levels of homocysteine the folic acid supplementation, induced the reduction of migraine attacks in 6 children and in 10 patients did not occur migraine attacks [23]. High levels of homocysteine are associated with vascular and endothelial damage and with cerebrovascular and cardiovascular disease; in particular, it has been assumed that hyperhomocysteinemia is involved in reducing brain blood flow and producing the depolarization wave defined as CSD. These facts associated with reduced oxygen transfer could act as trigger of migraine attack. The endothelial damage induced by high levels of homocysteine could decrease nitric oxide release and leads to the initiation and maintenance of migraine attacks [24]. In our previous study [25] the percentage of mutated migraine patients, both heterozygous and homozygous, is higher than that of patients with tension headache. The rate of mutated homozygous migraineurs is significantly higher than that of tension headaches, but HcY levels are not increased [25]. The common C677T polymorphism is associated with other pathologies. Numerous studies have shown the association between mutated MTHFR and migraine, especially with aura [24] and, in particular, the allele T and the TT genotype. Therefore, the role of folic acid metabolism in the pathogenesis of the headache seems relevant. Various studies, both in the population and in headache centers, have shown that migraine patients suffer from various comorbidities, particularly neurological, psychiatric and cardiovascular. The study of comorbidities of migraine is extremely important from the pathogenetic point of view as well as diagnostic and therapeutic. It is also important to remember the correlation between mutation of MTHFR and neural tube defects, with increased spontaneous abortion and congenital malformations. It is now clear that this mutation involves the presence of low levels of folic acid and high levels of homocysteine. Hyperhomocysteinemia is a cause of endothelial dysfunction, trigeminal dysfunction and alteration of blood coagulability. The trigeminal cell injury causes inflammation with dilation of the brain vessels that are the basis of the migraine pain. High levels of homocysteine therefore increase predisposition to migraine. The endothelial oxidative damage, through the formation of superanions, leads to an increase in the likelihood of migraine and other vascular disorders, such as stroke. All this has been confirmed by the fact that the folic acid supplement lowers homocysteine levels and reduces the frequency and severity of migraine [23,24]. Although the genetic condition and the resulting plasma abnormalities alone are insufficient to exacerbate the complex pathogenesis of organic lesions and migraine pathology, the allele may offer new possibilities for research and deeper investigation. Currently, migraine treatment is based on the administration of medications designed to manage and contain paroxysmal symptoms. In fact, although most times the therapy allows mitigating the size and frequency of abscess episodes, many patients are not completely immune to rather debilitating complications. The hypothesis of introducing treatment strategies capable of interrupting chronic disease and of avoiding, at least in part, the irreversible outcome of encephalopathy, could lead to the substitution or integration of today's predominantly symptomatic therapies [23-26]. The optimization of neurological practice, rather than responding to a purely scientific need for progress, would imply a dramatic improvement in the quality of life of patients, consistent with the most noble and authentic purposes for which medicine has been established.
Objective of Study
Aim of the study was the evaluation of one MTHFR gene mutation in a group of patients suffering from primary headache.
Materials and Methods
The study population was enrolled in the Department of Neuropsychiatric offering to Regional Headache Center of Region Abruzzo, University of L’Aquila. The patients were recruited according to temporal criteria by observations sequentially during the years 2015 and 2016. The children are born in Italy from parents Italians. The diagnosis of headache was made according to ICHD-III criteria [1].
The sample of study consists of 226 patients, 116 females and 110 males, aged between 11 to 13 years. Whole blood samples were collected using EDTA tubes and transferred in an upright position to local lab, where were stored at -20°C to freezer. During patient diagnostic evaluation we detected C677T variant of Metylenetetrahydrofolate Reductase based on the Real- Time Polymerase Chain Reaction (RT- PCR), we considered the homozygous and heterozygous T alleles of variant. The polymorphism C677T was identified using SAMPLE PREP thrombo kit (Diatech Pharmacogenetics), using a TAQman probe and a melting analysis to determinate genotypes [9]. The Total Homocysteine (tHcY) was determinated using a Chemiluminescent Immunoenzymatic Assay (CMIA) Homocysteine Architect [10].
Statistical Analysis
The statistical analysis was conducted by SAS system. The MTHFR polymorphism and tHcy levels were analized by χ2 test. The results were considered statistically significant when p ≤ 0.5.
Results
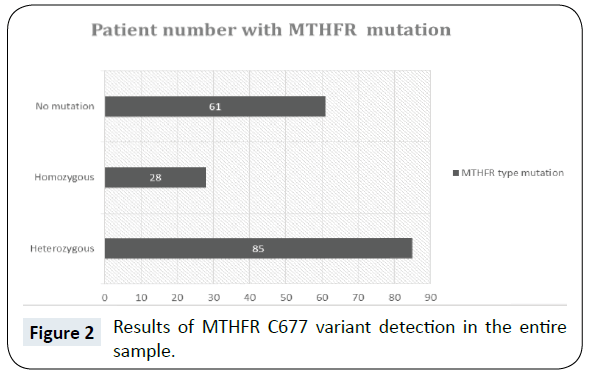
In the Figure 1 we show the diagnoses of primary headache and relative percentages of total sample. 96 patients suffering from Migraine without aura (MwA), 39 from Migraine with aura (MA), 40 from Tension type headache (TTH), 18 from Chronic TTH, 13 from chronic Migraine, 12 from other migraine. The C677T variant of MTHFR was detected in 147 patients (65%) Figure 2. The heterozygosis was present in the 50, 56% of females and 47, 06% of males, whereas the homozygosis 19, 10% of females and 12, 94% of males respectively. On the basis of headache diagnosis, in all migraineurs, the mutation was 67% compared to tension type patients in which was 33% (χ2 18,9, p<0,0001) Figure 3.
Homozygosis was identified in a rate of 70% for migraineurs and 30% of patients with tension type headache Figure 4. Also this comparison resulted statistically significant (p=0, 0030). In a percentage of 8.67% were observed increased levels of tHcY. In Table 1 we reported the results of statistical analysis of tHcY levels.
| Number | Mean | Standard Deviation | Median Values | Minimum | Maximum |
|---|---|---|---|---|---|
| 226 | 10 | 3.7 | 9.5 | 3.8 | 37.1 |
Table 1: Homocystein serum values (Mean +/- SD μmo/l).
Discussion
Our results confirmed available data of literature. In fact, in present study, population migraineurs had higher prevalence of MTHFR mutation, especially in homozygous. According to Lea [2] and other several Authors [6,11] C677T MTHFR variant predisposes to a susceptibility to migraine with aura and not to that without aura, in particular, it is well- known for European Caucasian population. A meta-analisys of 15 case control studies showed a close correlation between TT variant and Caucasians suffering from MA, in non-Caucasian population this genotype is associated with total migraine [11].
In a study of Bottini et al. was analyzed the prevalence of homozygous variant of an Italian study population, it was identified a trend towards an increased incidence of migraine in subjects who carried the mutation [8]; another study examined a group of Croatian pediatric patients suffering from migraine, the 677TT genotype was associated with an higher risk of migraine [11]. No association was found in Spanish and Portuguese migraineurs with TT genotype [10,12,13] Lorenz et al. analyzed MTHFR 677TT polymorphism in a group of Estonian pediatric migraineurs and did not detect a close link with migraine prevalence [14] MTHFR C677T variant differently occur in different countries, so it could be considered an ethnic-specific polymorphism [14]. In the pathogenesis of aura migraine is hereby accepted the concept of “cortical spreading depression (CSD)”, in the aura phase there is a cerebral oligoemia that starts to spread anteriorly and a condition of hyperemia follows to this [15,16]. It is known that about 37% of migraineurs presents white matter abnormalities, predominantly in the posterior circulation and in cerebellar territory; some case studies show a prevalence of these lesions (61%) in people with aura migraine and female [17]. Among the causes of such anomalies, in addition to the patent foramen ovale, high blood pressure and other risk factors for stroke, such as cigarette smoking, use of oral contraceptives, obesity, hypercholesterolemia, there is the MTHFR C677T mutation. An elevated plasma vWF activity is associated with MTHFR TT genotypes, so it represents a connection between migraine and ischemic predisposition [12,17].
In our study Hcy levels were normal in a greatest percentage of patients; these results are in contrast with available data in literature. A case study conducted in pediatric patients suffering from stroke, confirms the presence of hyperhomocysteinemia among the causal factors of stroke [18-20]. According to recent publications, migraine headache, especially those with aura, however, it could be conceived as the pathological mediator between the mutation and the phenotypic outcome of stroke [11,12]. In a study of Lea et al. on 52 patients suffering from aura migraine with increased levels of homocysteine, it was administered to patients vitamin supplementation, as homocysteine levels decreased frequency and severity of migraine attacks declined [21]. In the trial of Di Rosa et al. on 16 children with MA and high levels of homocysteine the folic acid supplementation, induced the reduction of migraine attacks in 6 children and in 10 patients did not occur migraine attacks [22]. High levels of homocysteine are associated with vascular and endothelial damage and with cerebrovascular and cardiovascular disease; in particular, it has been assumed that hyperhomocysteinemia is involved in reducing brain blood flow and producing the depolarization wave defined as CSD. These facts associated with reduced oxygen transfer could act as trigger of migraine attack. The endothelial damage induced by high levels of homocysteine could decrease nitric oxide release and leads to the initiation and maintenance of migraine attacks [23].
Conclusion
In our study the frequency of migraine is higher than that of tension headache (71.83% versus 28.17%), in particular, the percentage of mutated migraine patients, both heterozygous and homozygous, is higher than that of patients with tension headache (66.67% versus 33.33%). The rate of mutated homozygous migraineurs is significantly higher than that of tension headaches (70.37% vs. 29.63%). In our patients HcY levels are not increased. The common C677T polymorphism is associated with other pathologies. Numerous studies have shown the association between mutated MTHFR and migraine, especially with aura [22-24] and, in particular, the allele T and the TT genotype. Therefore, the role of folic acid metabolism in the pathogenesis of the headache seems relevant. Various studies, both in the population and in headache centers, have shown that migraine patients suffer from various comorbidities, particularly neurological, psychiatric and cardiovascular. The study of comorbidities of migraine is extremely important from the pathogenetic point of view as well as diagnostic and therapeutic. It is also important to remember the correlation between mutation of MTHFR and neural tube defects, with increased spontaneous abortion and congenital malformations. It is now clear that this mutation involves the presence of low levels of folic acid and high levels of homocysteine. Hyperomocysteinemia is a cause of endothelial dysfunction, trigeminal dysfunction and alteration of blood coagulability. The trigeminal cell injury causes inflammation with dilation of the brain vessels that are the basis of the migraine pain. High levels of homocysteine therefore increase predisposition to migraine. The endothelial oxidative damage, through the formation of super anions, leads to an increase in the likelihood of migraine and other vascular disorders, such as stroke. Folate deficiency is associated with the destruction of nucleic acids, slow repair of DNA, and increased chromosomal rupture. All this has been confirmed by the fact that the folic acid supplement lowers homocysteine levels and reduces the frequency and severity of migraine [22-23]. Although the genetic condition and the resulting plasma abnormalities alone are insufficient to exacerbate the complex pathogenesis of organic lesions and migraine pathology, the allele may offer new possibilities for research and deeper investigation. Currently, migraine treatment is based on the administration of medications designed to manage and contain paroxysmal symptoms. In fact, although most times the therapy allows mitigating the size and frequency of abscess episodes, many patients are not completely immune to rather debilitating complications. The hypothesis of introducing treatment strategies capable of interrupting chronic disease and of avoiding, at least in part, the irreversible outcome of encephalopathy, could lead to the substitution or integration of today's predominantly symptomatic therapies [22-26]. The optimization of neurological practice, rather than responding to a purely scientific need for progress, would imply a dramatic improvement in the quality of life of patients, consistent with the most noble and authentic purposes for which medicine has been established.
References
- International headache society (2013) The international classification of headache disorders, 3rd edition (Beta version) Cephalalgia 33: 6.
- Lea RA, Ovcaric M, Sundholm J, MacMillan J, Griffiths LR (2004) The ethylenetetrahydrofolate reductase gene variant C677T influences susceptibility to migraine with aura. BMC Med 12: 2-3.
- Frosst P, BlomH J, Milos R (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10: 111-113.
- Moll S, Varga EA (2015) Homocysteine and MTHFR Mutations. Circulation 132: 6-9.
- Gasparini CF, Sutherland HG, Griffiths LR (2013) Studies on the pathophysiology and genetic basis of migraine. Curr Genomics 14: 300-315.
- Kowa H, Yasui K, Takeshima T, Urakami K, Sakai F, et al. (2000) The homozygous C677T mutation in the methylenetetrahydrofolate reductase gene is agenetic risk factor for migraine. Am J Med Genet 96: 762-764.
- Lee MJ, Lee C, Chung CS (2016) The Migraine-Stroke Connection Stroke 18: 146-56.
- Bottini F, Celle ME, Calevo MG, Amato S, Minniti, et al. (2006) Metabolic and Genetic Risk Factors for Migraine in Children. Cephalalgia 26: 731-737.
- Endler G, Kyrle PA, Eichinger S, Exner M, Mannhalter C (2001) Multiplexed mutagenically separated PCR: simultaneous single-tube detection of the factor V R506Q (G1691A), the prothrombin G20210A, and the methylenetetrahydrofolate reductase A223V (C677T) variants. Clin Chem 47: 333-335.
- Refsum H, Grindflek AW, Ueland PM, Fredriksen A, Meyer K, et al. (2004) Screening for serum total homocysteine in newborn children. Clin Chem 50: 1769-1784.
- Oterino A, Valle N, Bravo Y, Munoz P, Sanchez-Velasco P, et al. (2004) MTHFR T677 Homozygosis. Influences the Presence of Aura in Migraineurs. Cephalalgia 24: 491-494.
- Rubino E, Ferrero M, Rainero I, Binello E, Vaula G, et al. (2009) Association of the C677T polymorphism in the MTHFR gene with migraine: a meta-analysis. Cephalalgia 29: 818-825.
- Herak DC, Antolic MR, Krleza JL, Pavic M, Dodig S, et al. (2007) Inherited Prothrombotic Risk Factors in Children With Stroke, Transient Ischemic Attack, or Migraine. Pediatrics. 123: e653-e660.
- Ferro A, Castro MJ, Lemos C, Santos M, Sousa A, et al. (2008) The C677T Polymorphism in MTHFR is Not Associated with Migraine in Portugal. Disease Markers 25: 107-113.
- Anna LL, Tiina K, Evelin M, Tiit N (2014) Are Methylenetetrahydrofolate Reductase (MTHFR) Gene Polymorphisms C677T and A1298C Associated with Higher Risk of Pediatric Migraine in Boys and Girls? J Biomed Sci Eng 7: 464-472.
- An XK, Lu CX, Ma QL, Zhang XR, Burgunder JM, et al. (2013) Association of MTHFR C677T Polymorphisms with Susceptibility to Migraine in the Chinese Population. Neuroscience Letters 549: 7800-8115.
- Sadeghi O, Maghsoudi Z, Askari G, Khorvash F, Feizi A (2014) Association between serum levels of homocysteine with characteristics of migraine attacks in migraine with aura. J Res Med Sci 19: 1041-1045.
- Del Sette M, Dinia L, Bonzano L, Roccatagliata L, Finocchi C, et al. (2008) White matter lesions in migraine and right-to-left shunt: a conventional and diffusion MRI study. Cephalalgia 28: 376-382.
- Tietjen GE, Al-Qasmi MM, Athanas K, Utley C, Herial NA (2007) Altered hemostasis in migraineurs studied with a dynamic flow system. Thromb Res 119: 217-222.
- Paonessa A, Limbucci N, Tozzi E, Splendiani A, Gallucci M (2010) Radiological strategy in acute stroke in children. Eur J Radiol 74: 77-85.
- Tietjen GE, Al-Qasmi MM, Athanas K, Utley C, Herial NA (2007) Altered hemostasis in migraineurs studied with a dynamic flow system. Thromb Res 119: 217-222.
- Lea R, Colson N, Quinlan S, Macmillan J, Griffiths L (2009) The effects of vitamin supplementation and MTHFR (C677T) genotype on homocysteine-lowering and migraine disability. Pharmacogenet Genomics 19: 422-428.
- Di Rosa G, Attinà S, Spanò M, Ingegneri G, Sgrò DL, et al. (2007) Efficacy of folic acid in children with migraine, hyperhomocysteinemia and MTHFR polymorphisms. Headache 47: 1342-1344.
- Menon S, Lea RA, Ingle S (2015) Effects of dietary folate intake on migraine disability and frequency. Headache 55: 301-319.
- Tozzi E, Piccorossi A, Gammella C, Onofri A, Verrotti A, et al. (2017) The Methylenetetrahydrofolate Reductase (Mthfr) Gene Polymorphism C677t and the Migraine: Evaluation of a Children Sample. Int J Neurol Dis 1: 028-032.
- Todt U, Freudenberg J, Goebel I, Netzer C, Heinze A, et al. (2006) MTHFR C677T polymorphism and migraine with aura. Ann Neurol 60: 621-624.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences